Recombinant DNA cloning is used in a variety of synthetic biology applications, such as vaccine design, bioremediation, research models, and so much more. DNA cloning involves generating recombinant DNA by introducing DNA fragments into vector constructs. The recombinant DNA construct is then transfected into host cells to express the genes or open reading frames (ORFs), which are then used for the intended application.
gBlocks™ Gene Fragments are synthetic, double-stranded DNA fragments that are a customizable and flexible alternative to PCR products for a variety of cloning methods. Unlike using PCR to generate construct inserts, these synthetic DNA fragments allow for assembly of genes of interest into vectors without requiring an original DNA template. They also allow for easy customization like codon optimization or adding common flanking regions such as transcription elements or vector homology.
Gateway cloning is an alternative cloning method that doesn’t rely on restriction enzymes, but instead uses specific vector types that contain homologous sites for transferring DNA between vectors. This article provides an overview of Gateway cloning, its limitations, and considerations for your experimental design.
Overview of Gateway Cloning
The Gateway cloning system is a fast and efficient recombination-based cloning technique commercialized by Invitrogen/Thermo Fisher Scientific [1]. The technique uses recombinases derived from bacteriophage lambda to transfer DNA between vectors by homologous (att) sites. This is a reversible, site-specific recombination reaction using proprietary enzyme mixes, Clonases, with Gateway entry and destination vector types. These vectors contain antibiotic resistance genes that are used, once the fragment of interest is cloned in, for positive selection. Inversely, the toxin‑producing ccdB gene is for negative selection, removing constructs that have not undergone effective recombination so only clones with desired DNA fragments are viable.
The LR Clonase™ is the forward reaction mediating the recombination between the entry clone containing attL sites and the exchange of the ccdB gene flanked by the attR sites on the destination vector.
The BP Clonase™ is the reverse reaction which is facilitated between the Expression clone and the Donor vector.
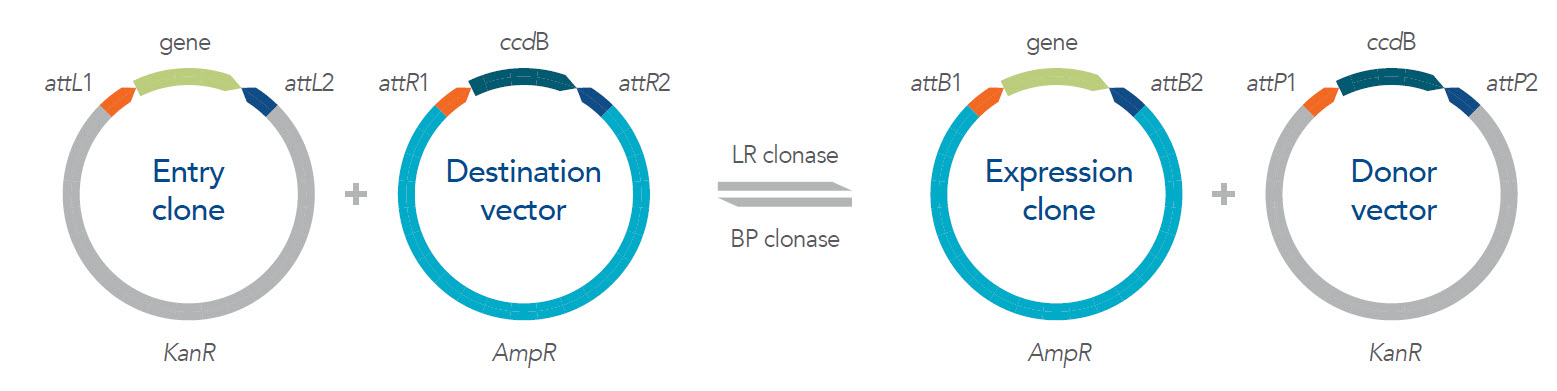
Here is a brief explanation of the function and roles of each clone and vector used in the Gateway cloning system as shown in Figure 1:
Entry clone
The entry clone contains a kanamycin resistance gene (positive selection marker) and the target gene or fragment of interest flanked by attL sites that are homologous to the attR sites on the destination vector. If moving in the forward direction, the entry clone is typically created through simple TOPO cloning of your fragment of interest into the entry vector.
Destination vector
There are a variety of available destination vectors with different protein expression systems, depending on the host cell type. In addition to components required to express your gene of interest in the proper host, the destination vector contains an inducible negative selection marker, ccdB, which encodes for a microbial toxin, flanked by the attR recombination sites. This allows for the DNA fragment, coding for the gene of interest, from the Entry clone to exchange with the ccdB gene in the destination vector. The destination vector contains an AmpR gene, which is a positive selection marker encoding for ampicillin resistance.
Donor vector
If the goal is to create a protein expression construct for your gene of interest, the donor vector is the final construct that should be removed from the reaction by inducing expression of the ccdB gene to select out clones with that construct. If moving in reverse, the donor vector becomes the Entry clone containing the ccdB gene (negative marker) flanked by the attP sites and KanR gene (positive selection marker).
Expression clone
The expression clone contains the DNA fragment encoding the protein to be expressed, which is flanked by the attB and contains the KanR (positive selection marker). By adding both a kanamycin antibiotic and inducing the ccdB gene, the expression clone should be the final construct that can then move into the downstream expression experiments in the preferred host cells.
Gateway Cloning protocol tips and tricks
Limitations
Gateway recombinant technology is a helpful cloning option as protocols are relatively straightforward and cloning multiple fragments into a wide variety of expression vectors makes it easy to move genes between systems. However, Gateway cloning has a few drawbacks, including the necessary “scar” att regions, which are not present in other scarless methods like Golden Gate cloning and Gibson Assembly® (Telesis Bio) [2]. Also, specific vectors and Clonase enzyme mixes add cost for each recombination reaction.
Design considerations
gBlocks™ and gBlocks HiFi Gene Fragments are double-stranded DNA fragments that can be used for your restriction enzyme, Gibson Assembly, Golden Gate, and Gateway (Invitrogen) cloning protocols. Easily design your high-fidelity IDT gene fragments here.
When designing your DNA fragments consider the following:
- If planning to use recombination to create your entry clone, attB-flanking sites will need to be added to the ends of the DNA fragments. For this, it’s important to also include necessary protein expression elements for your host (start and stop codons, ribosome recognition sequences) and be aware of how reading frames will be affected.
- For TOPO-cloning into Gateway entry vectors, using fragments directly (through PCR with Taq polymerase or gBlocks Gene Fragments) is sufficient. Additional entry vectors are available for other cloning methods.
For more tips for working with gBlocks Gene Fragments, see this article or for more design considerations and futher information about cloning methods, download the DNA Cloning Guide.

